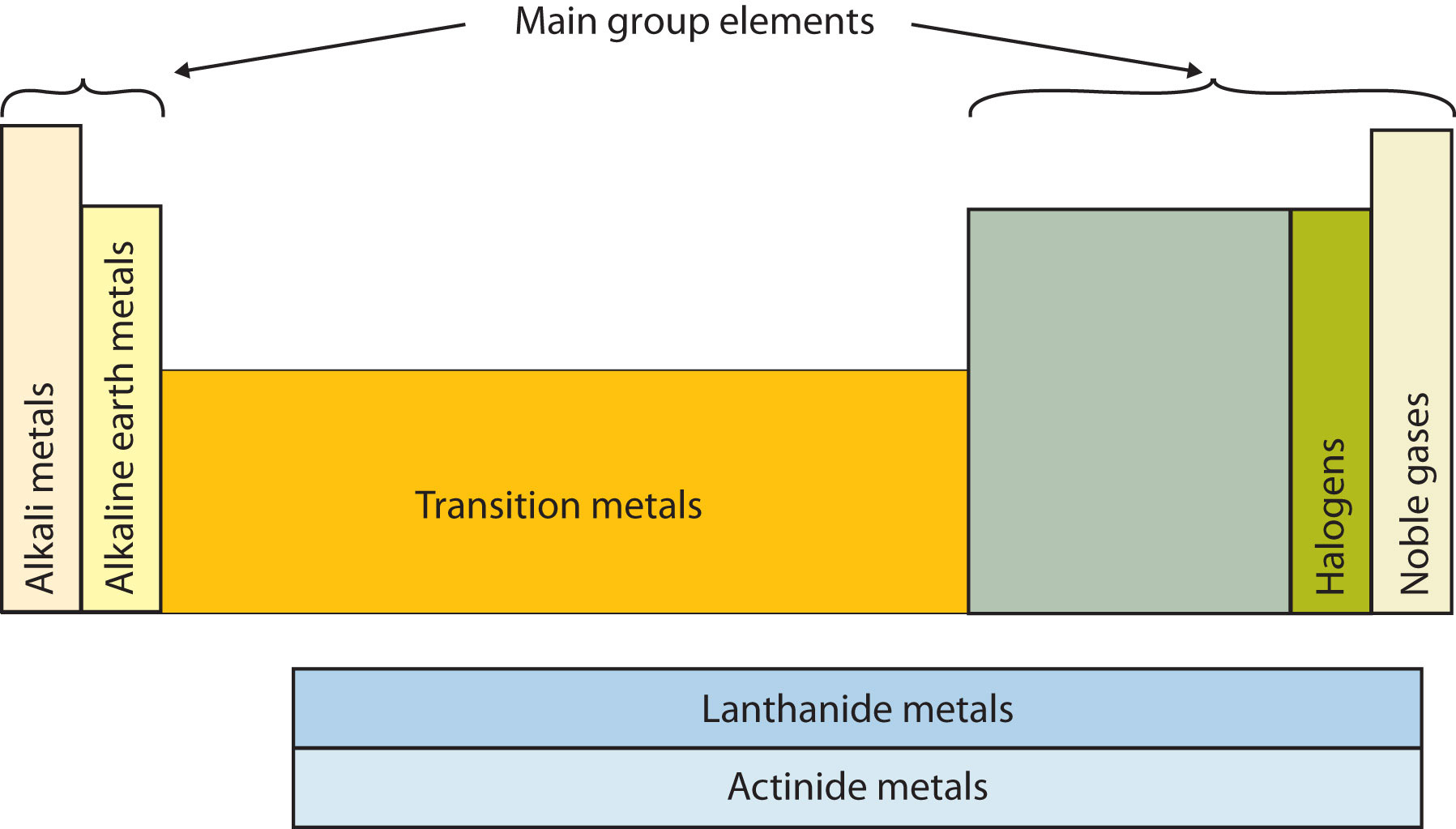
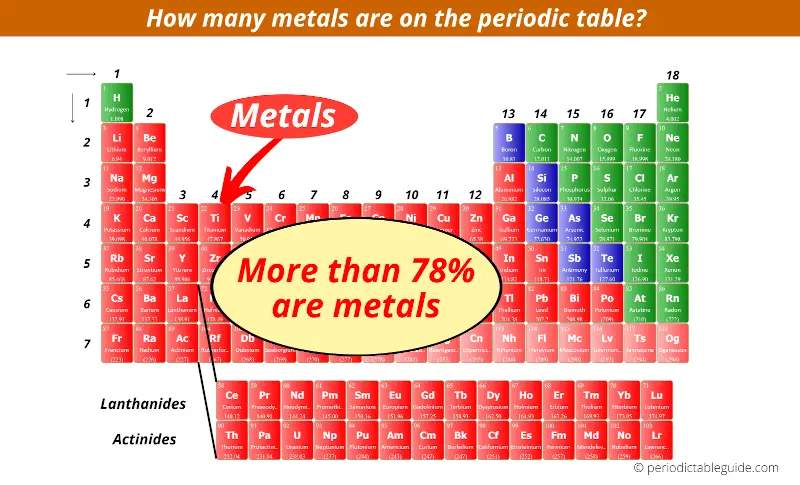
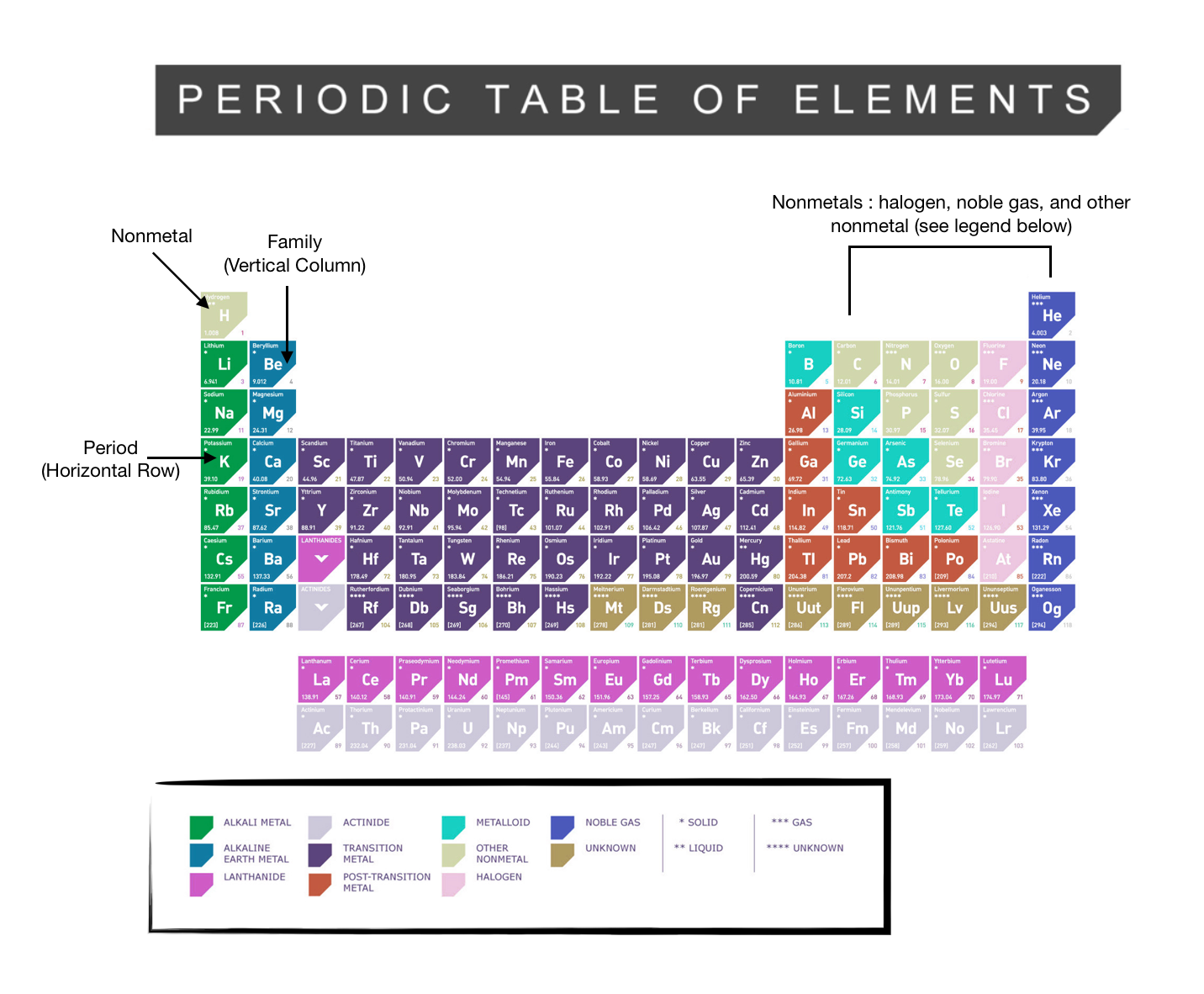
44 periodic table with metal and nonmetal labels
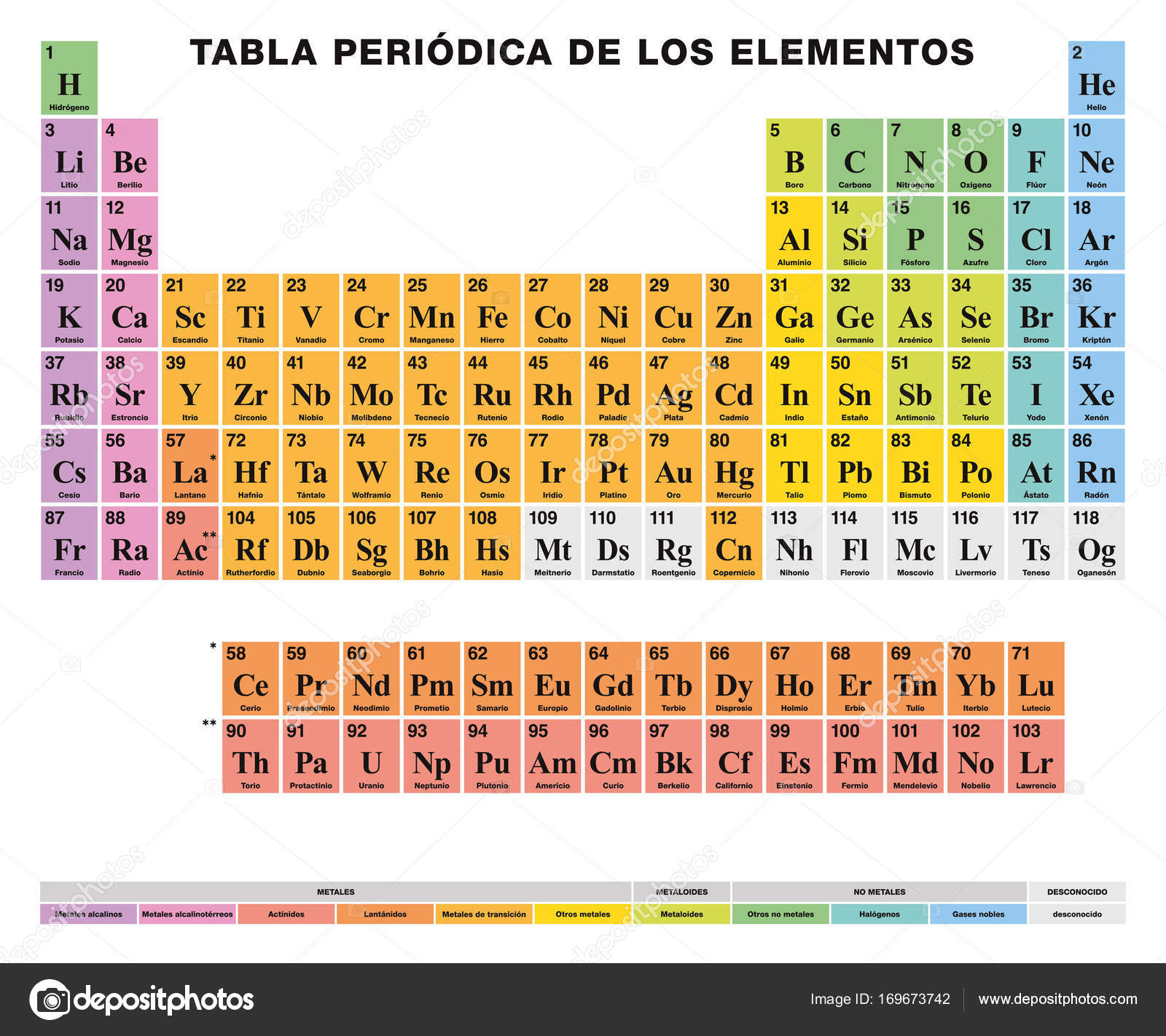
First 10 elements of the periodic table - chemistry1science 07.08.2021 · First 10 elements of the periodic table First 10 elements and their symbols: Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron... › rennen30 › integrated-scienceIntegrated Science Module for Grade 7 -- Quarter 1-2 - SlideShare Aug 13, 2014 · Hence, metals and nonmetals, being elements, may form compounds. Combining with oxygen, a metal or a nonmetal may form an oxide. However, the acidity differs depending on the nature of this oxide. This, again, is a defining characteristic of a metal and a nonmetal. A metal oxide is generally basic; while a nonmetal oxide is acidic.
› 39234782 › Lecture_Notes_inLecture Notes in General and Inorganic Chemistry Part 1 TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants, reductants, acids, bases, metal ions, etc. Titration is based on a reaction between the analyte (unknown sample) and the regent of known concentration and reaction stoichiometry.
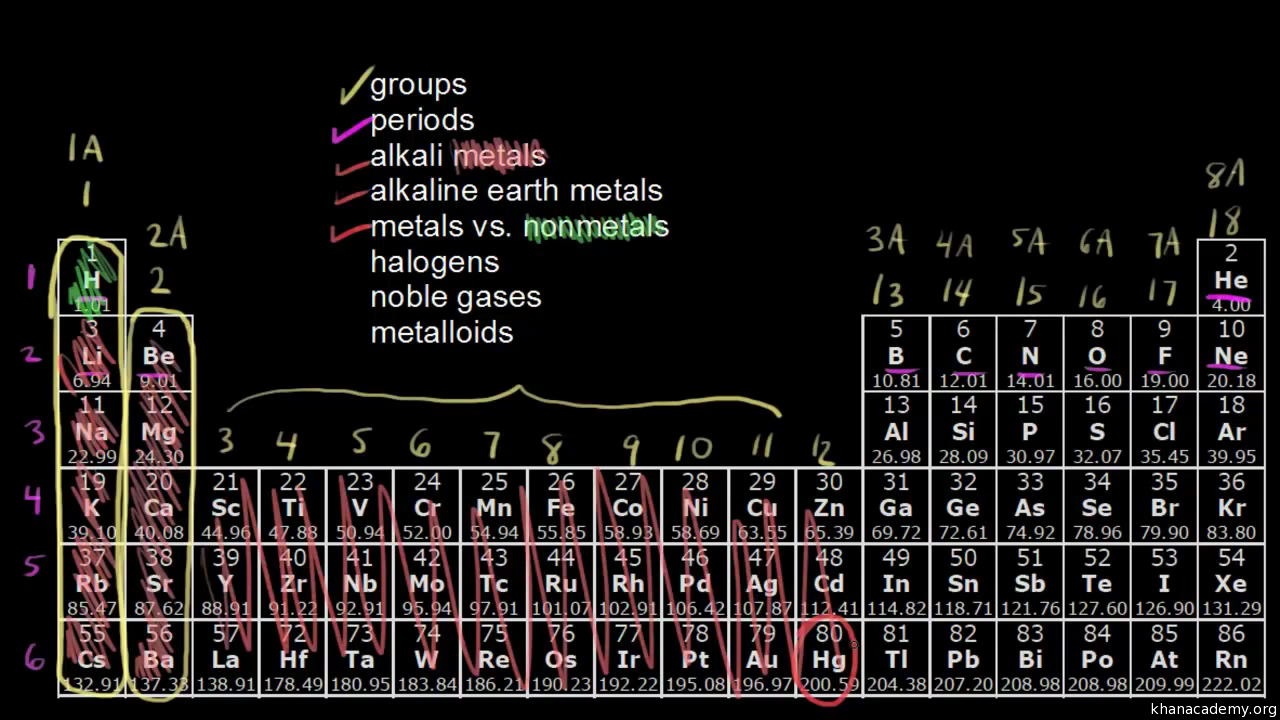
Periodic table with metal and nonmetal labels
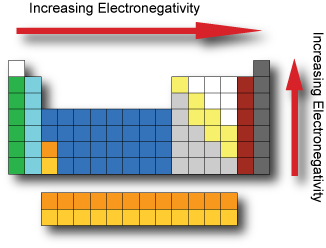
Lecture Notes in General and Inorganic Chemistry Part 1 Lecture notes in General and Inorganic Chemistry provides an introduction to the chemistry of inorganic molecules. The emphasis is on basic principles of atomic and molecular structure, thermodynamics, chemical kinetics and catalysis, properties of solutions, acid-base equilibria, hydrolysis and buffer solutions, and coordination compounds. quizlet.com › 160124557 › chem-ch-3-chem-ch-2-flashChem Ch 3, Chem Ch 2 Flashcards | Quizlet A main group metal tends to lose electrons, forming a cation with the same number of electrons as the nearest noble gas in the periodic table. A main group nonmetal tends to gain electrons, forming an anion with the same number of electrons as the nearest noble gas. The various groups gain or lose electrons as summarized in the following table: Chem Ch 3, Chem Ch 2 Flashcards | Quizlet If you are not given electronegativity values, you can still predict the bond type using the periodic table. Metals have low electronegativity compared to nonmetals. So in general, we can predict that any metal-nonmetal combination will be ionic and any nonmetal-nonmetal combination will be covalent. If electronegativity values aren't given, you should assume that a covalent bond is …
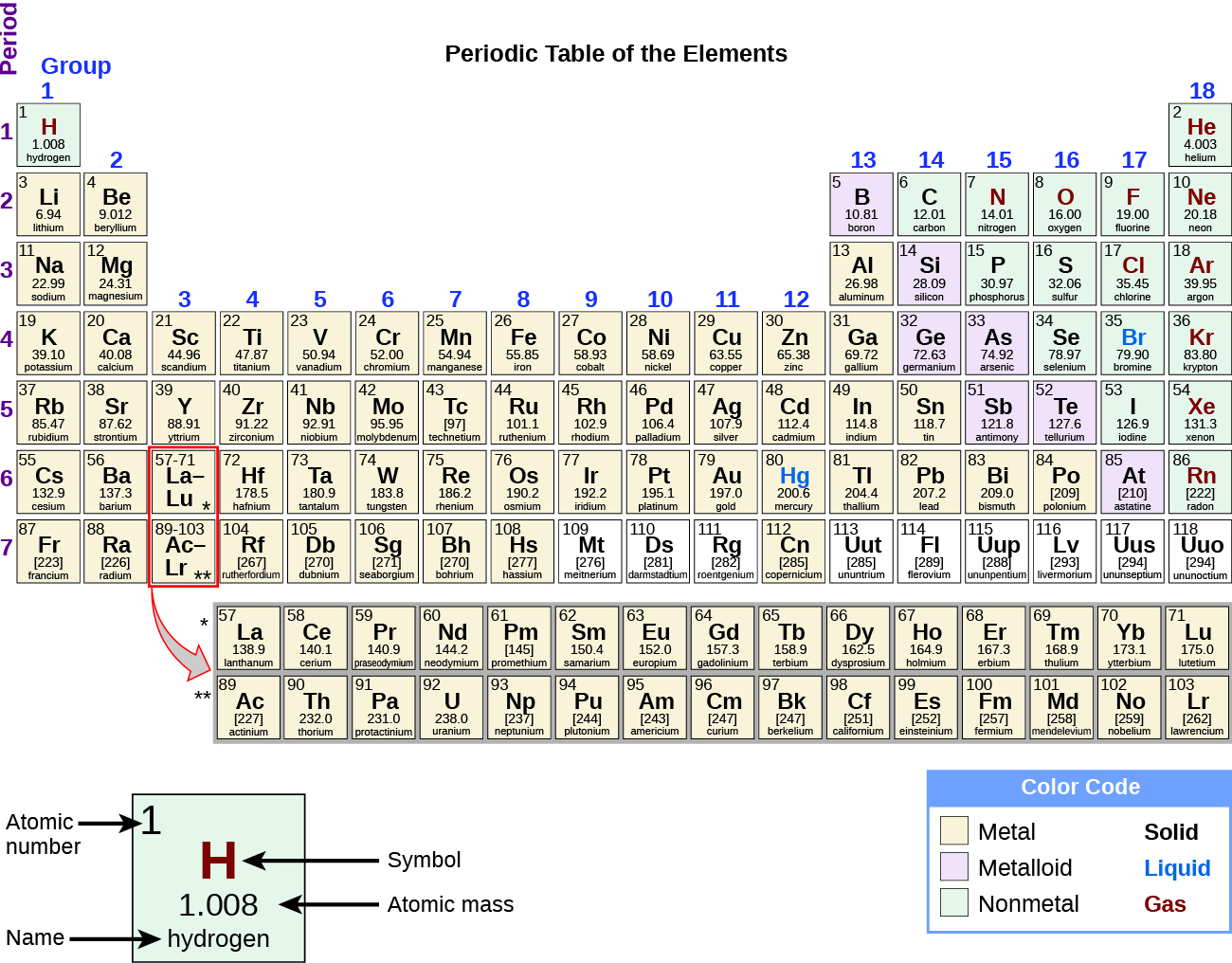
Periodic table with metal and nonmetal labels. en.wikipedia.org › wiki › Periodic_tablePeriodic table - Wikipedia The periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number. Elements are placed in the periodic table by their electron configurations , [17] which exhibit periodic recurrences that explain the trends of ... Metal and non-metal oxides - The periodic table - BBC Metals and non-metals can be heated in oxygen to make compounds called oxides. Find out more with BBC Bitesize. For students between the ages of 11 and 14. › 2021 › 08First 10 elements of the periodic table - chemistry1science Aug 07, 2021 · First 10 elements of the periodic table . First 10 elements and their symbols: Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron (B) Carbon (C) Nitrogen (N) Oxygen (O) Fluorine (F) Neon (Ne) 1- Hydrogen. Symbol: H. Atomic Number: 1. Atomic Mass: 1.008. Oxidation States: +1, -1. Standard State: Gas. Group Block: Nonmetal. Year ... Periodic table - Wikipedia The periodic table, also known as the periodic table of the (chemical) elements, is a tabular display of the chemical elements.It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic …
en.wikipedia.org › wiki › NonmetalNonmetal - Wikipedia In periodic table terms, an analogy can be drawn between the noble gases and noble metals such as platinum and gold, with the latter being similarly reluctant to enter into chemical combination. As a further example, xenon, in the +8 oxidation state, forms a pale yellow explosive oxide, XeO 4 , while osmium , another noble metal, forms a yellow ... › lhoralight › k-to-12-grade-7K TO 12 GRADE 7 LEARNING MATERIAL IN SCIENCE (Q1-Q2) - SlideShare Mar 11, 2014 · Material Needed periodic table of elements Procedure 1. Every element has a name. In each box of the table, you will find only one name. One box corresponds to one element. Using the partial figure of the periodic table on the right, find where oxygen is. 37. Grade 7 Science: Matter 33 Diversity of Materials in the Environment 2. Integrated Science Module for Grade 7 -- Quarter 1-2 - SlideShare 13.08.2014 · Thus, there is no need to memorize such table. A periodic table is provided at the end page of Modules 3 and 5. The information placed there is limited to the scope of the module for this quarter. It is highly encouraged to begin with the names and symbols of the elements as they try to know what the elements are. Group number will be introduced at the latter part of … Chem Ch 3, Chem Ch 2 Flashcards | Quizlet If you are not given electronegativity values, you can still predict the bond type using the periodic table. Metals have low electronegativity compared to nonmetals. So in general, we can predict that any metal-nonmetal combination will be ionic and any nonmetal-nonmetal combination will be covalent. If electronegativity values aren't given, you should assume that a covalent bond is …
quizlet.com › 160124557 › chem-ch-3-chem-ch-2-flashChem Ch 3, Chem Ch 2 Flashcards | Quizlet A main group metal tends to lose electrons, forming a cation with the same number of electrons as the nearest noble gas in the periodic table. A main group nonmetal tends to gain electrons, forming an anion with the same number of electrons as the nearest noble gas. The various groups gain or lose electrons as summarized in the following table: Lecture Notes in General and Inorganic Chemistry Part 1 Lecture notes in General and Inorganic Chemistry provides an introduction to the chemistry of inorganic molecules. The emphasis is on basic principles of atomic and molecular structure, thermodynamics, chemical kinetics and catalysis, properties of solutions, acid-base equilibria, hydrolysis and buffer solutions, and coordination compounds.

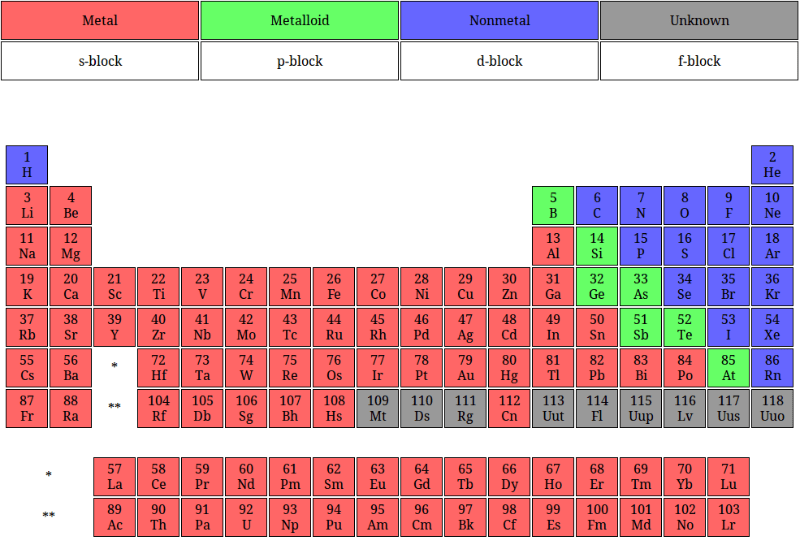

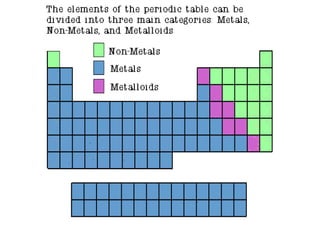



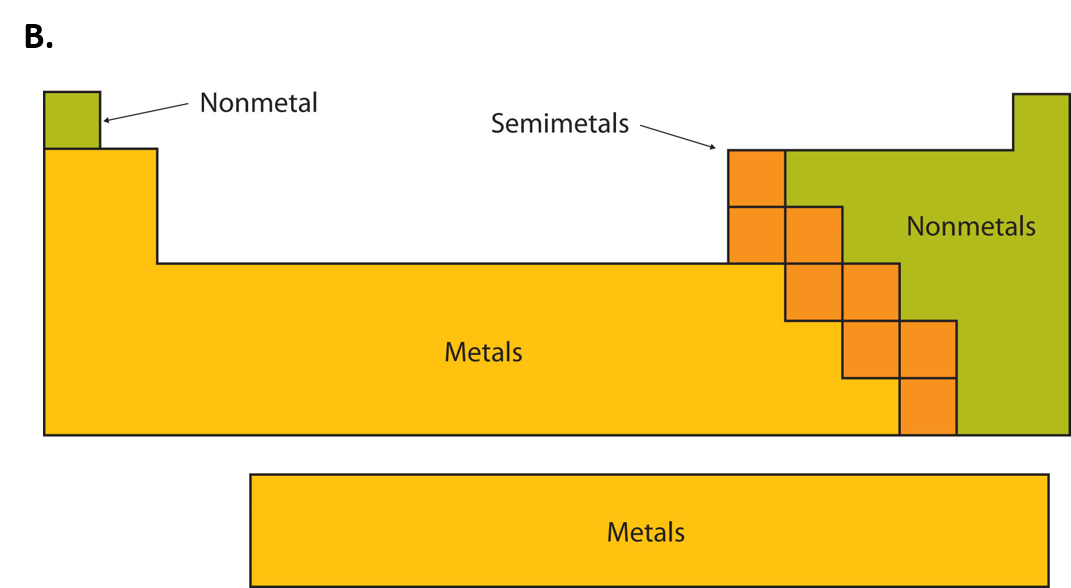











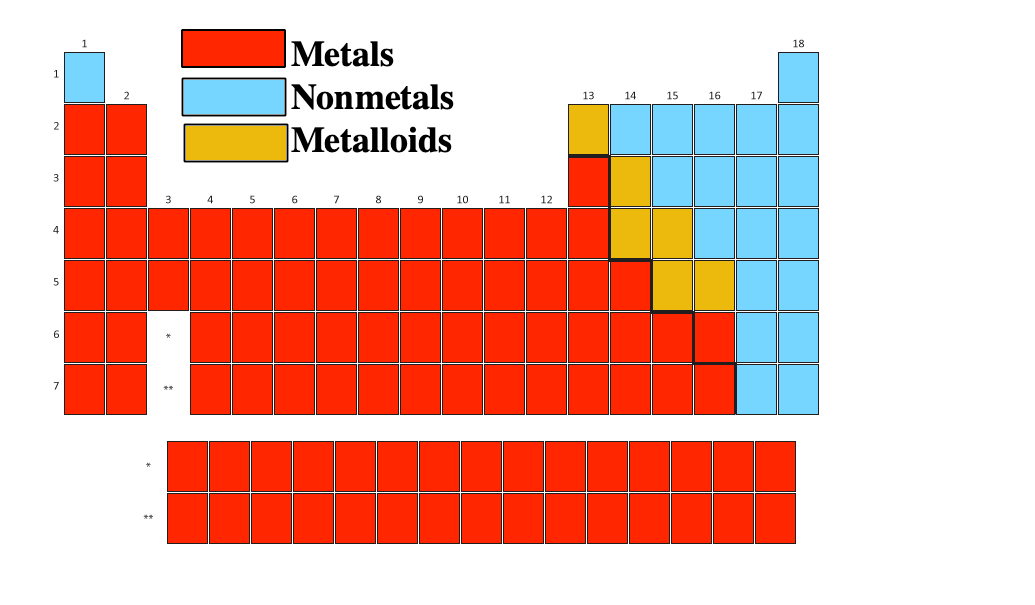

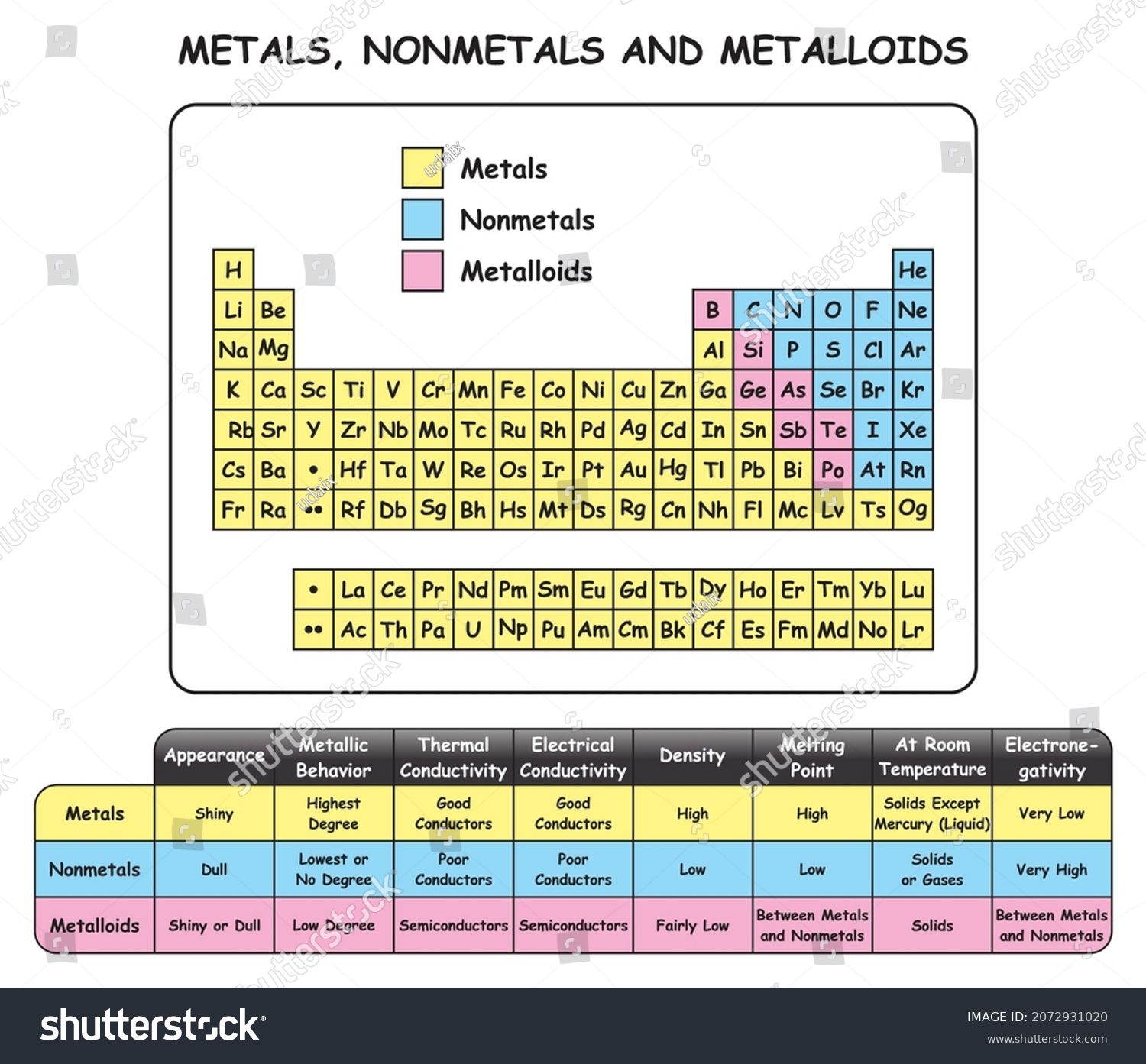



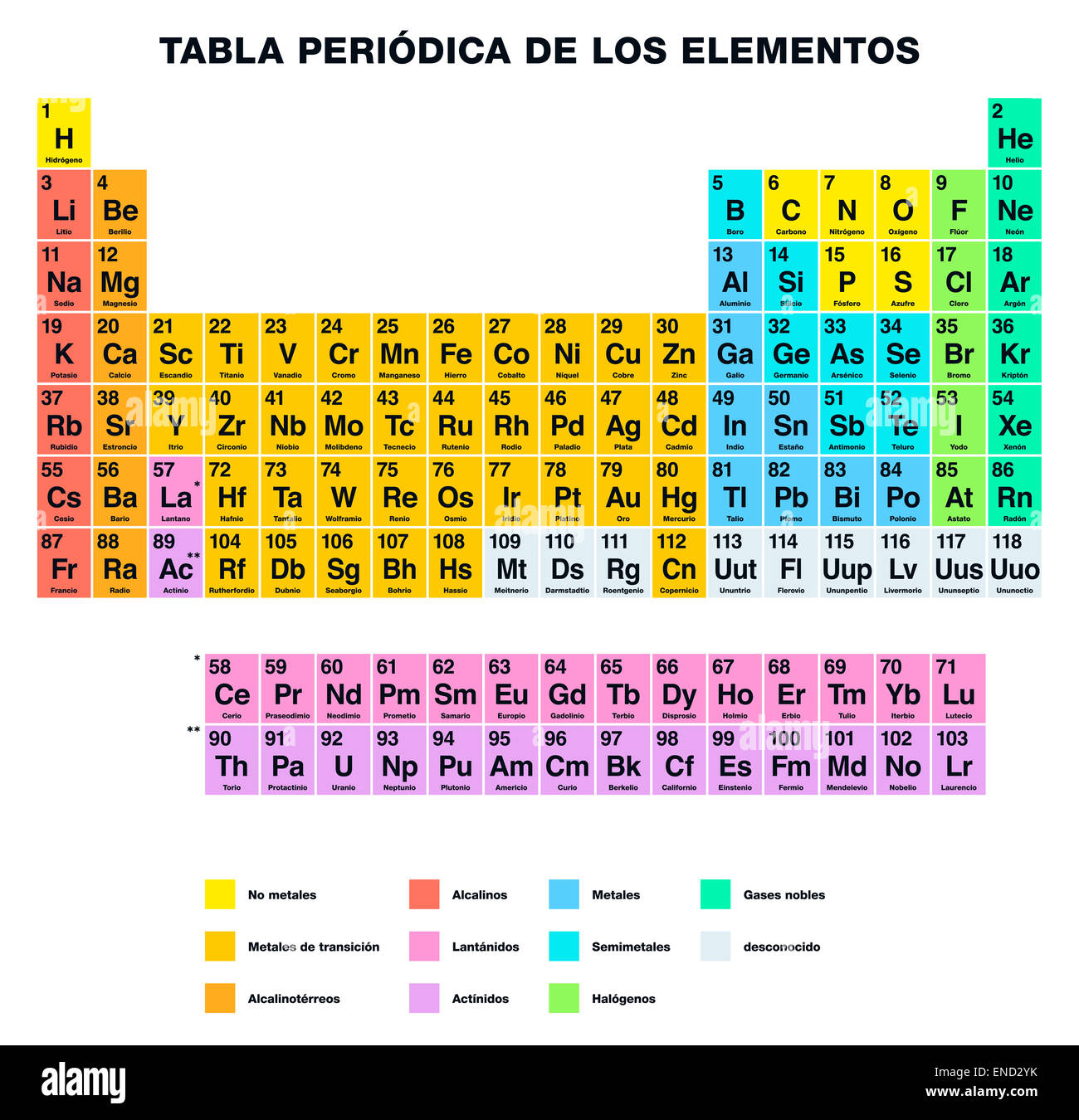
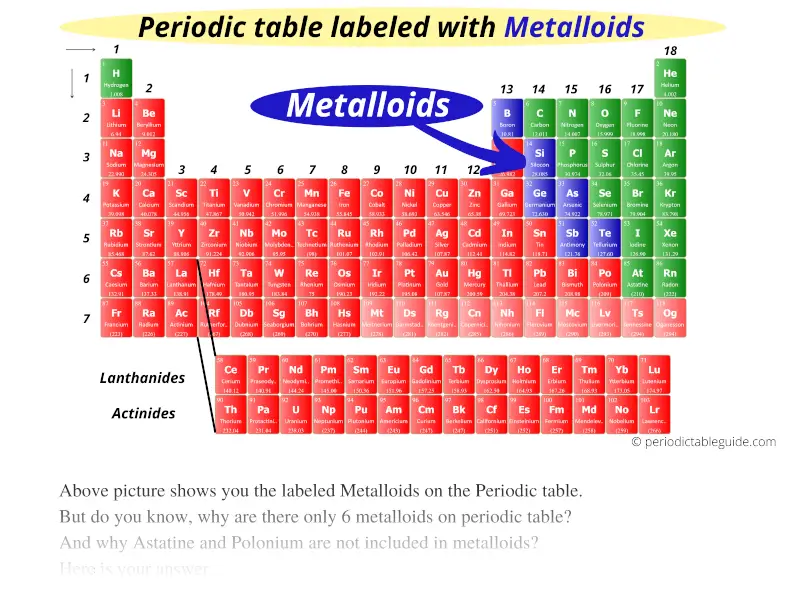
Post a Comment for "44 periodic table with metal and nonmetal labels"